Retroperitoneal Fibrosis Medication (NovRF)
Pipeline
NovRF
Program Target
Retroperitoneal Fibrosis
Patient Numbers
0.27 Million
Development Stage
Clinical Phase I/II
NovMetaPharma develops NovRF, a retroperitoneal fibrosis medication of first-in-class new substance.
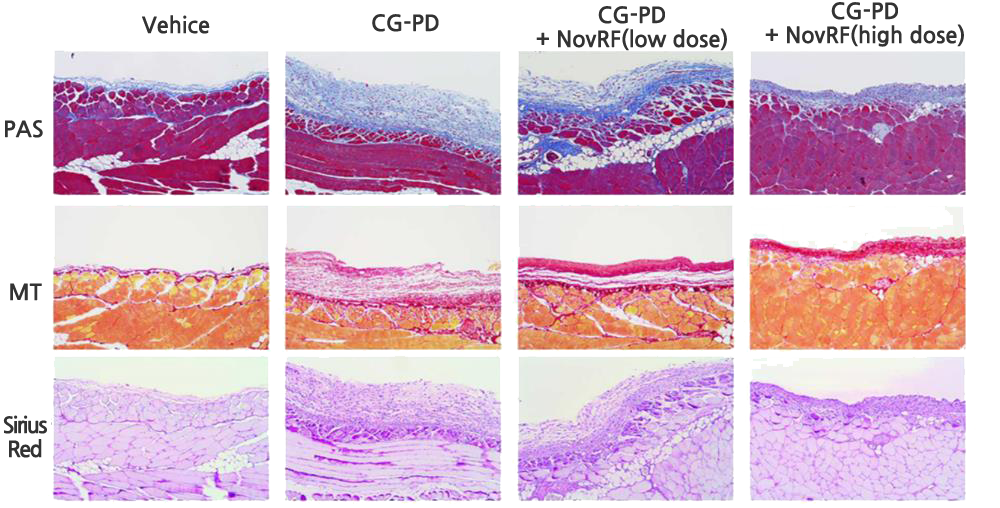
The effect of inhibiting peritoneal EMT (epithelial-to-mesenchymal transition), peritoneal cell death, or immune cell invasion into the peritoneum has been confirmed with NovRF (retroperitoneal fibrosis medication) in an animal model in which retroperitoneal fibrosis was induced.
This confirms NovRF’s treatment effect for retroperitoneal fibrosis. Based on this, NovMetaPharma is conducting joint R&D to develop new retroperitoneal fibrosis prevention, improvement, and medication.
 Outline
Outline
 Diabetes Medication
Diabetes Medication 
 Anti-obesity Medication
Anti-obesity Medication 
 Kidney Disease Medication
Kidney Disease Medication 
 Fibrosis Medication
Fibrosis Medication 
 Retroperitoneal Fibrosis Medication
Retroperitoneal Fibrosis Medication