Kidney Disease Medication (NovRD)
Pipeline
NovRD
Program Target
Renal Disease
Patient Numbers
697 Million
Development Stage
Clinical Phase I/II
NovMetaPharma develops NovRD, a chronic kidney disease medication of first-in-class new substance.
The direct medication for chronic kidney disease available is only symptom relief and complication prevention through hypertension medication. Therefore, the market demand for direct chronic kidney disease medication is very high.
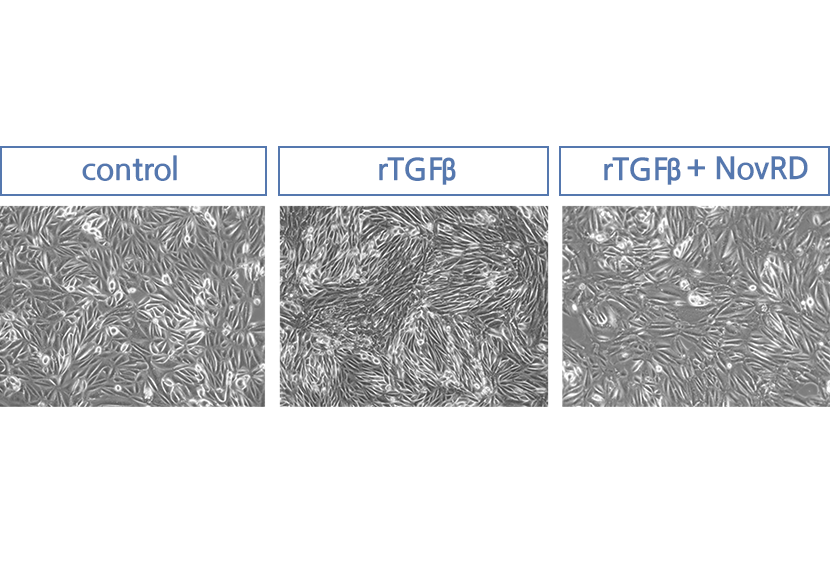
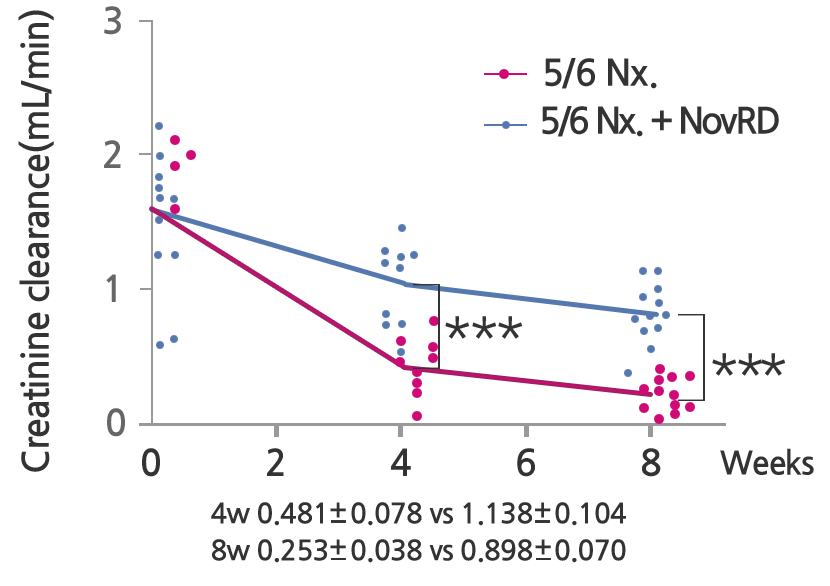
NovRD is a first-in-class drug that directly treats chronic kidney disease and has proven its excellent ability to inhibit kidney cell death and inflammation.
Moreover, under the new drug approval process, systematic benefits such as expedited evaluation can be obtained in cases such as chronic kidney disease that are without existing medications.
Accordingly, NovRD’s new drug development and commercialization schedule is expected to be further accelerated and NovMetaPharma is accelerating the development of NovRD currently
01
Inhibition of cell death
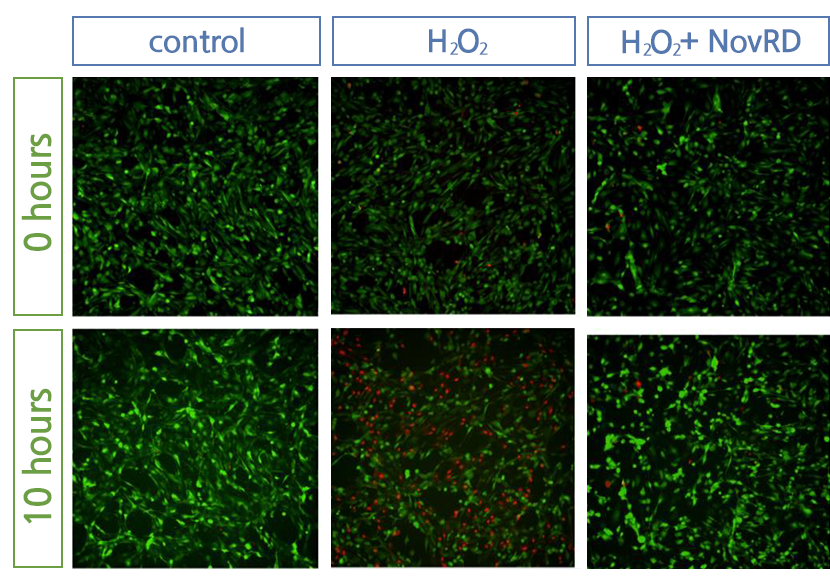
Cell death has been confirmed to be effectively inhibited when NovRD was administered.
02
Inhibition of inflammation
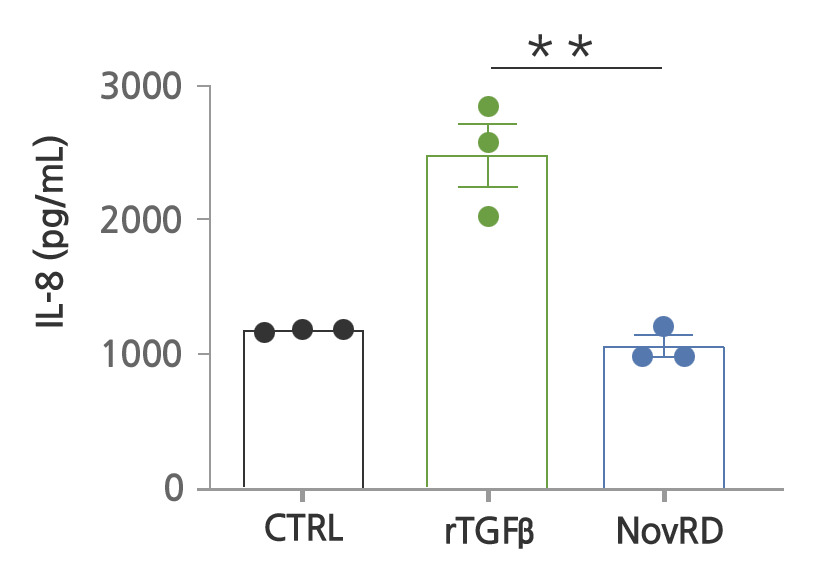
Inflammation has been confirmed to be significantly reduced when NovRD was administered.
03
Expedited evaluation
With the high possibility of being designated as Fast Track by the US FDA, the approval of the new drug is expected to proceed quickly
 Outline
Outline
 Diabetes Medication
Diabetes Medication 
 Anti-obesity Medication
Anti-obesity Medication 
 Kidney Disease Medication
Kidney Disease Medication 
 Fibrosis Medication
Fibrosis Medication 
 Retroperitoneal Fibrosis Medication
Retroperitoneal Fibrosis Medication