Fibrosis Medication (NovFS)
Pipeline
NovFS
Program Target
Fibrosis
Patient Numbers
5 Million
Development Stage
Clinical Phase (I)/II
NovMetaPharma develops NovFS, a (pulmonary, hepatic, etc.) fibrosis medication of first-in-class new substance.
NovMetaPharma has identified excellent anti-inflammatory and anti-fibrotic efficacies in NovFS (fibrosis medication) cell and animal testing. This confirmed the possibility of developing NovFS as fibrosis medication, and based on this, NovMetaPharma is conducting joint R&D of NovFS.
Treatment effects of idiopathic pulmonary fibrosis
Decrease of major mechanism gene expression causing idiopathic pulmonary fibrosis
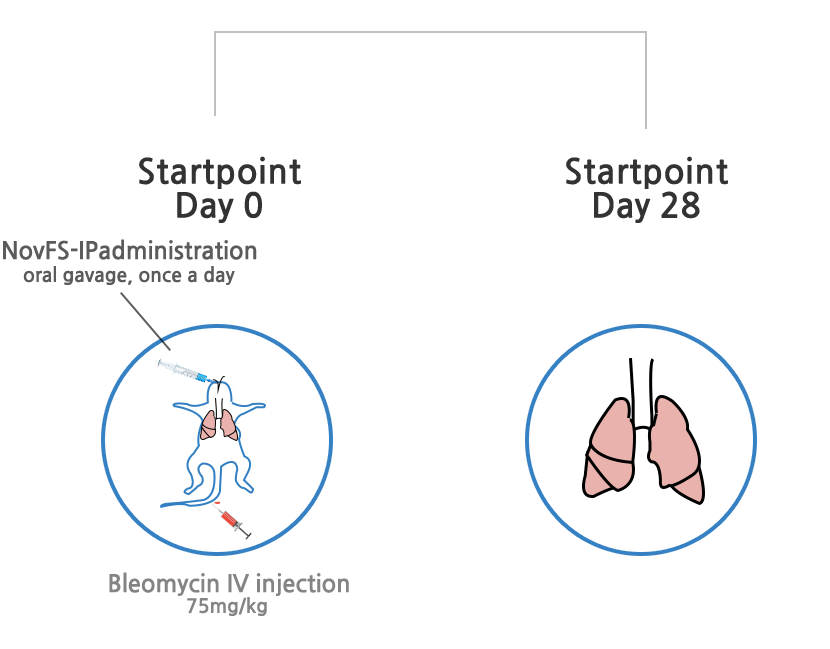
Decrease of mediator genes (ECM) causing idiopathic pulmonary fibrosis
 Outline
Outline
 Diabetes Medication
Diabetes Medication 
 Anti-obesity Medication
Anti-obesity Medication 
 Kidney Disease Medication
Kidney Disease Medication 
 Fibrosis Medication
Fibrosis Medication 
 Retroperitoneal Fibrosis Medication
Retroperitoneal Fibrosis Medication