Diabetes Medication (NovDB2)
Pipeline
NovDB2
Program Target
Type 2 Diabetes
Patient Numbers
629 Million
Development Stage
Clinical Phase II
NovMetaPharma develops NovDB2, an insulin sensitivity improving agent of first-in-class new substance.
Insulin Sensitivity Improvement Function
Currently, most of the type 2 diabetes medication market is insulin injection or simple blood sugar control drugs, which do not have the effect of improving insulin sensitivity, the underlying cause of diabetes. In the case of the TZD series, the only commercially available insulin sensitivity improving agent, not only risks of cardiovascular disease and bladder cancer but also side effects of weight gain have been identified.
On the other hand, NovDB2 has been confirmed to improve insulin sensitivity in a clinical trial by the US FDA. Also, the superior safety of NovDB2 has been verified in clinical trials such as chronic toxicity and reproductive toxicity conducted in the same clinical trial. On top of such insulin sensitivity improvement function and safety, NovDB2 has proven its unrivaled weight control effect as well.
As a groundbreaking insulin sensitivity improving agent that will change the game in the diabetes medication market, NovDB2 will be presented as a clear solution to the global demand for fundamental diabetes medication.
01
Insulin resistance improvement effect
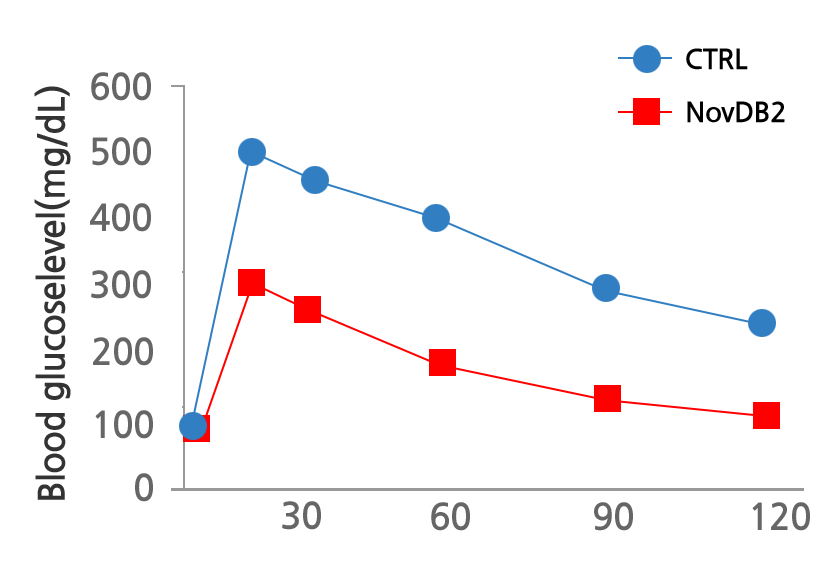
The trend of improving insulin sensitivity was confirmed in indicators related to type 2 diabetes treatment such as HbA1c, fasting blood sugar, and insulin and glucose tolerance by the US FDA clinical phase 2 (including a and b).
02
Safety
Safety such as chronic toxicity and reproductive toxicity has been verified through several global clinical and non-clinical trials.
03
Weight loss
Significant G114 reduction in weight has been confirmed through the US FDA clinical phase 2 (including a and b).
 Outline
Outline
 Diabetes Medication
Diabetes Medication 
 Anti-obesity Medication
Anti-obesity Medication 
 Kidney Disease Medication
Kidney Disease Medication 
 Fibrosis Medication
Fibrosis Medication 
 Retroperitoneal Fibrosis Medication
Retroperitoneal Fibrosis Medication